This series of posts is a followup to the project that Dr. Eugene Fine and I described in our campaign at Experiment.com. as follow-up to Dr. Fine’s pilot study of ten advanced cancer patients on ketogenic diets and the in vitro projects that we are carrying out in parallel.
The last post described the two major processes in energy metabolism, (anaerobic) glycolysis and respiration. Pyruvate is the product of glycolysis and has many fates. (Remember pyruvate and pyruvic acid refer to the same chemical). For cells that rely largely on glycolysis, pyruvate is converted to several final products like ethanol, lactic acid and a bunch of other stuff that microorganisms make in the fermentation of glucose. (The unique smell of butter, e.g., is due to acetoin and other condensation products of pyruvate). Rapidly exercising muscles also produce lactic acid.
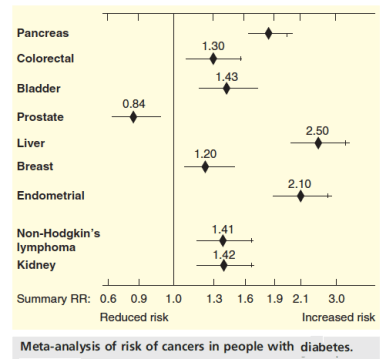
The sudden interest in the metabolic approach to cancer stems from the work of Otto Warburg whose lab in the 1930’s was a center for the study of metabolism. (Hans Krebs was an Assistant Professor in the lab). Warburg’s landmark observation was that cells from cancer tissue showed a higher ratio of lactate to CO2 than normal cells, that is, the cancerous tissue was metabolizing glucose via glycolysis to a greater degree than normal even though oxygen was present. The Coris (Carl and Gerty of the Cori cycle) demonstrated what is now called the Warburg effect in a whole animal. Ultimately, Warburg refined the result by comparing the ratio of lactate:CO2 in a cannulated artery to that in the vein for a normal forearm muscle. He compared that to the ratio in the forearm of the same patient that contained a tumor. Warburg claimed that this greater dependence on glycolysis was a general feature of all cancers and for a long time it was assumed that there was a defect in the mitochondrion in cancer cells. These are both exaggerations but aerobic glycolysis appears as a feature of many cancers and defects in mitochondria, where they exist, are more subtle than gross structural damage. The figure shows current understanding of the Warburg Effect.

What about this mechanism makes us think that ketone bodies are going to be effective against cancer? We need one more step in biochemical background to explain what we think is going on. In normal aerobic cells, pyruvate is converted to the compound acetyl-CoA. Acetyl-CoA represents another big player in metabolism and functions as the real substrate for aerobic metabolism. If you have taken general chemistry, you will recognize acetyl-CoA as a a derivative of acetic acid.
The reaction acetyl-CoA ➛ 2CO2 is the main transformation from which we get energy. Acetyl-CoA provides the building block for fatty acids and for ketone bodies. Conversely, fatty acids are a fuel for cells because they can be broken down to acetyl-CoA. Under conditions of starvation, or a low-carbohydrate diet, the liver assembles 2 acetyl-CoA’s to ketone bodies (β-hydroxy butyrate and acetoacetyl-CoA). The ketone bodies are transported to other cells where they are disassembled back to acetyl-CoA and are processed in the cell for energy. The liver is a kind of metabolic command center and ketone bodies are a way for the liver to deliver acetyl-CoA to other cells.
Now we are at the point of asking how a cell knows what to do when presented with a choice of fuels? In particular, how does the input from fat dial down glycolysis so that pyruvate, which could be used for something else (in starvation or low-carb, it will be substrate for gluconeogenesis), is not used to make acetyl-CoA. It turns out that acetylCoA (that is, fat or ketone bodies) regulate their own use by feeding back and directly or indirectly turning off glycolysis (in other words: don’t process pyruvate to acetyl-CoA because we already have a lot). The feedback system is known as the Randle cycle and appears (roughly) as the dotted line in our expanded metabolic scheme.
![]() Where we are going. In our earlier work Dr. Fine and I and our assistant, Anna Miller, found that if we grow cancer cells in culture, acetoacetate (one of the ketone bodies) will inhibit their growth and will reduce the amount of ATP that they can generate. Normal cells, however, are not inhibited by ketone bodies and the cells may even be using them. Our working explanation is that the ketone bodies are inhibiting the cancer cell through the Randle cycle. Now, normal cells can maintain energy, that is compensate for the Randle cycle, by running the TCA cycle (in fact, that is the purpose of the Randle cycle: to switch fuel sources). The cancer cells, however, have some kind of defect in aerobic metabolism and can’t compensate. How does this happen? That’s what we’re trying to find out but we have a good guess. (A good guess in science means that when we find out it’s wrong we’ll probably see a better idea). We find that our cancer cells in culture over-express a protein called uncoupling protein-2 (UCP2). We think that’s a player. To be discussed in Part IV.
Where we are going. In our earlier work Dr. Fine and I and our assistant, Anna Miller, found that if we grow cancer cells in culture, acetoacetate (one of the ketone bodies) will inhibit their growth and will reduce the amount of ATP that they can generate. Normal cells, however, are not inhibited by ketone bodies and the cells may even be using them. Our working explanation is that the ketone bodies are inhibiting the cancer cell through the Randle cycle. Now, normal cells can maintain energy, that is compensate for the Randle cycle, by running the TCA cycle (in fact, that is the purpose of the Randle cycle: to switch fuel sources). The cancer cells, however, have some kind of defect in aerobic metabolism and can’t compensate. How does this happen? That’s what we’re trying to find out but we have a good guess. (A good guess in science means that when we find out it’s wrong we’ll probably see a better idea). We find that our cancer cells in culture over-express a protein called uncoupling protein-2 (UCP2). We think that’s a player. To be discussed in Part IV.