The series of posts Ketogenic Diets for Cancer follows from the experiment.com campaign run by Dr. Eugene J. Fine and myself. The campaign is now over and we were most grateful for the support and wanted to keep the discussion going. Currently on this site we will try to summarize and organize some of the exchanges. Use the comments section if you have questions for me or Dr. Fine. We expect the discussion to be broad but the two key papers are Dr. Fine’s pilot study with ten advanced cancer patients which, though a small study, may still be the only prospective human study, and a related in vitro study.
To follow up on the previous post, the potential of the ketogenic diet derives from a change in basic outlook from the genetic approach to the metabolic approach. In our original discussion on experiment.com, several people thought that the explanation of the metabolism was too technical. Here wepresent a simplified version that may allow easier access to the main ideas.
Energy exchange in biochemistry is represented in the interconversion of the molecules known as ADP and ATP, the former the “low energy” form and the latter, the “high energy” form. In essence, it costs you energy to make ATP from ADP and, if you have ATP, the energy from going back to ADP can be used to do work, usually chemical work, making something new like protein or DNA. (The quotation marks remind us that the energy is in the reaction not in the molecules as such). In a rough sort of way then the energy charge of the cell is identified with the level of ATP.
Two major processes, glycolysis and respiration, provide energy as ATP. Glycolysis, common to almost all living cells, converts glucose into a three carbon compound pyruvic acid (or pyruvate — acids have two different forms and the names are used interchangeably in biochemistry). Glycolysis does not require oxygen and is referred to as anaerobic metabolism. Pyruvate is a key metabolite and can be converted to many substances. Some cells, rapidly exercising muscle, red blood cells and some microorganisms are restricted to anaerobic metabolism and the final product from pyruvate is lactate (lactic acid).
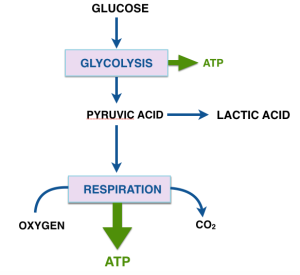
The second method, respiration is aerobic and can convert all the carbons in pyruvate to CO2 and water. Most mammalian cells carry our respiration and process pyruvic acid aerobically. Respiration is more efficient, produces more ATP than glycolysis, although glycolysis is faster — related to its role in rapidly exercising muslce. Respiration is dependent on oxygen and produces most of the ATP in aerobic cells. You probably know the punch line here: cancer cells are more likely to rely on glycolysis than the normal cells of which they are variants even if there is oxygen present. What Warburg original measured was the ratio of lactic acid to CO2 and this represents a good indication of the cancerous state.
The Warburg effect calls attention to the choice of fuel for cellular metabolism as a key in understanding cancer. Closing in on the question of why we think ketone bodies are important, we have to look at other inputs to energy metabolism. Fat is obviously the major contributor. The fatty acids supplied by ingested and stored lipid goes directly into respiration. Under conditions of starvation or of carbohydrate restriction, the fatty acids can also provide the material for synthesis of ketone bodies. Ketone bodies, in turn, derived from fats provide an alternative fuel in place of glucose for many cells. Ketone bodies are made in the liver and transported to other cells, notably the brain, for energyy. (Looking ahead to more detailed explanation, the derivative of acetic acid, acetyl-CoA is the actual input to respiration; the ketone bodies supply acetyl-CoA to other cells). The figure summarizes the basic ideas on energy metabolism.
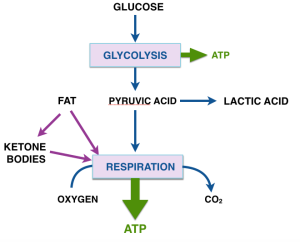
We found that if you grow cancer cells in culture, ketone bodies will inhibit their growth and the amount of ATP that they can generate. Next post will describe the experments and how we think they might be explained by the metabolic pathway in the figure.
Dr Rosedale believes mTOR is a key player in cancer. Is that being measured?
mTOR (Mammalian target rapamycin) is a key player in many aspects of metabolism and we intend to measure changes in association with other parameters. Rapamycin and other compounds that can inhibit mTOR can have anti-cancer effects but they are not predictable and in practice are frequently too toxic. Our perspective, however, is that mTOR is stimulated by many things and targets many things and, most significantly can feedback on the stimuli that affects its production and modification. We are trying to take a top-down approach since we think that the different things that can happen with mTOR and other signaling molecules may show up as a consistent phenotype characterized by changes in metabolism.
A good review at http://jcs.biologists.org/content/joces/122/20/3589.full.pdf has a figure that shows the complexity of mTOR signaling.
[…] Ketogenic Diets for Cancer II. Background on why ketone bodies might help. […]