Guest post: Dr. Eugene J. Fine
Last time I discussed our pilot study showing the effects of carbohydrate (CHO) restriction & insulin inhibition (INSINH) in patients with advanced cancers. We described how the molecular effects of INSINH plus systemic (total body) effects like ketosis might inhibit cancer growth. My goal now is to present the underlying hypothesis behind the idea with the goal of understanding how patients with cancers might respond if we inhibited insulin’s actions? Should all patients respond? If not, why not? Might some patients get worse? These ideas were described briefly in our publication describing our pilot protocol.
The hypothesis hinges on understanding how natural selection works, and how it works on two levels. The first is based on our evolutionary heritage and how humans evolved biochemically. The second level addresses how cancers evolve within people. In sum, we’re considering an intersection of human evolution over our long prehistory vs. cancer evolution over several years within human beings. Let me explain:
Human evolution: We’ll arbitrarily date the first humans from the evolution of Homo erectus about 1 million years ago, or from Homo sapiens, approximately 200,000 years ago. Since agriculture emerged about 10,000 years ago we can conclude that our ancestors were hunter-gatherers for at least 95% of our existence. According to paleontological evidence dietary CHO of hunter-gathers was in the range of 20-35% of total caloric intake. For most of human evolution we had no bread on the table, nor cakes, puddings, pies or chips. Wild fruits gathered during hunter-gather days don’t measure up to the huge domesticated fruits now abundantly available all year round. It’s difficult to imagine how humans didn’t starve during the winters in northern Europe or on the Asian steppes. Even in Africa, the cooling climate beginning around 100,000 years ago forced migrations of people toward more hospitable (and food abundant) climes. At the very least, there’s little doubt that our forebears did not consume excess dietary sugar and starch resulting in obesity and metabolic syndrome. In short, for 95% of human existence, the paleontological record shows that we were very tolerant of minimal CHO intake.
But the best evidence is in our biochemistry: under well fed modern conditions our brain uses about 130 grams of glucose/day (for most of us in the developed world). During a fast our brain continues to use glucose derived from the breakdown of glycogen in our liver as well as from glucose synthesis (gluconeogenesis) largely from body proteins. However, the continued consumption of our own proteins for a fast lasting longer than a few days isn’t a good evolutionary survival strategy as the protein would be stolen from our muscles, killing us rapidly from heart and diaphragm failure.
A key survival strategy that allowed conservation of body protein — the ability to generate ketone bodies as an alternative fuel source — is what actually survived. Under conditions of fasting—total reduction in nutrients (including carbohydrate) — glucose concentration will fall in the bloodstream thereby causing less pancreatic insulin secretion. Breakdown of fat in fat cells (lipolysis) is ordinarily inhibited by high levels of insulin. Conversely, fat breakdown accelerates under conditions of fasting (low insulin signaling), causing release of fatty acids (FA) into the blood. FA’s circulate to the liver where they then get metabolized into circulating ketone bodies (KB). That’s why fasting results in ketosis, as does strict low carbohydrate dieting (e.g. less than 50 grams of CHO/day). The brain uses 130 grams of glucose/day under our ordinary modern dietary conditions. But during starvation/fasting, over a 6 week period, the brain switches to ketone bodies to supply more than 2/3 of its fuel. This masterful metabolic switch spares protein breakdown and permits humans to tolerate starvation as long as fat stores hold out. That’s why people can survive without food sometimes for months, whereas absolute water deprivation can kill in just a few days.
One way to describe the evolution of our metabolism is that, over millennia, environmental conditions selected those individuals for survival who a) stored up enough fat during the more plentiful months to provide an energy source for their body and b) had the enzyme capacity to make ketone bodies (KB) from the stored fat and could utilize these KB as brain fuel to reduce the brain’s need for glucose derived from protein.. These individuals survived over the long lean winters to reproduce, and they passed on the genes that enabled this capacity.
Anyway, civilization and agriculture changed the availability of many foods, but especially grains. Bread became the ‘staff of life.’ In recent years, excessive, cheap CHO, coupled with the Food Pyramid’s recommendations to consume CHO to the tune of 55-70% of total caloric intake have likely contributed to widespread metabolic syndrome, obesity, type 2 diabetes, dyslipidemias and hypertension. Finally, obesity and high insulin concentrations in the blood (both linked to dietary CHO excess) have now been associated with an increased risk for a variety of different cancers. Obesity has in fact surpassed smoking as the greatest behavioral risk factor for cancers in the U.S. Reducing cancer risk by CHO restriction would seem to be a meaningful preventive cultural dietary goal. The idea appears to resemble our study where we treated cancers with a VLC/INSINH diet, but it’s not quite the same thing. Cancer cells behave differently from normal cells, so, metabolically, treatment isn’t quite the same as prevention.
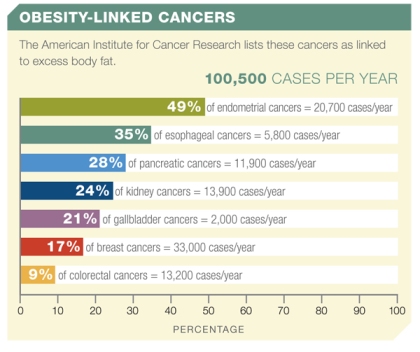
Figure 1: The American Institute for Cancer Research links more than 100,000 new cases of cancer per year to obesity.
Cancer evolution: Cancer initiation is a subject of intense research interest. I share the prevailing view that cancers start with a mutation that changes a normal cell into a slightly abnormal but not yet malignant cell. A total of four to six critical mutations over a period of 30 to 70 generational doublings taking 3 or more years result in unregulated cell growth, proliferation, invasion, metastasis, in other words cancer-like behavior (or ‘phenotype’) ().
Regardless of the initial insult, each one of these abnormal but not yet malignant cells must evolve over time within a human organism. We must remember that mutations in normal cells happen all the time and they are usually maladaptive or destructive to the cells; healthy cells have mechanisms to protect the organism by a kind of cell suicide or apoptosis, best thought of as self-pruning of maladaptive cells. So most mutations within these aberrant cells will cause cell death and will not result in a cancer. But there are mutations, seen in many types of cancers, which can cause resistance to apoptosis and therefore permit abnormal cell growth to continue.
This is where the evolution of humans and the evolution of cancers within humans collide. In the modern developed world most cancers evolve within individuals under conditions of substrate/nutrient abundance, and especially CHO abundance. And while famine continues to plague people during wars and in underdeveloped regions, it’s nevertheless reasonable to suggest that sustained ketosis and INSINH are not prevalent in the modern developed world. Therefore in the developed world, cancers in many people are unlikely to have experienced a microenvironment due to the metabolic effects of insulin inhibition (INSINH) or ketosis (a limiting state of INSINH).
In summary, INSINH would represent a new metabolic selective pressure for many cancers to which they would reasonably be vulnerable.
Hypothesis: Humans are adapted to starvation and the metabolically related low CHO diet/INSINH state. On the other hand, cancers in large cohorts of people in developed countries are under high chronic insulin stimulation and are largely unexposed to the unfamiliar INSINH state. These cancers will sensibly express a wide range of molecular and metabolic vulnerabilities to INSINH. However, they may express an equally wide range of adaptive mutations. Investigators have confirmed both vulnerability of some cancer types to inhibition by added ketone bodies as well as continued uninhibited cancer growth with added KBs in other cancers.
Two important corollaries about cancers in hunter-gatherers and modern ‘low carbers’:
First, there’s no a priori reason that cancers can’t arise in individuals on INSINH/VLC diets in the modern world, or in modern (or prehistoric) hunter-gathers. Cancers initiate from a series of mutations due to causes that may be intrinsic to cell metabolism or to the external environment. Even for hunter-gatherers, heavy metals in ground water (even without civilization), background radiation all around us, smoke from smoking tobacco or herbs, campfires and cooking (hunter gatherers had controlled fire for close to 1 million years) and chronic inflammation are some of the factors that have existed for millennia, even if more pervasive since the onset of civilization. Although anecdotal and paleontological evidence is all that we have, cancers have been reported in ancient bones. Nothing would prevent cancers from forming, even in the likely reduced insulin signaling state of early hunter-gatherers (or in modern people on VLC/INSINH diets).
Second, while fewer cancers might be expected to develop, those hunter-gatherers (or modern low-carbers) who do develop cancers would not experience a VLC diet as a new selective pressure. Our pilot study, in fact, excluded patients who had been on a VLC diet within 3 years of the diagnosis of their cancer because a VLC/INSINH diet would not be expected to have any positive effect on their cancers.
To sum up: For people on high CHO diets who develop cancer, a low carbohydrate diet targeting insulin (VLC/INSINH) may be therapeutic. The rationale is that control of insulin and the presence of ketone bodies provides a new selective evolutionary pressure to which the cancers may not be adapted. On the other hand, those cancers that develop in people who are already on low-carbohydrate diets (already in a state of dietary insulin inhibition) will not be expected to be vulnerable to the VLC/INSINH diet.
Natural Selection as the premise is a great idea. On the idea that VLC/INSINH would not result in a inhospitable environment for cancers that developed in that same environment today, using NS as the premise as well, we could hypothesize that back then, those who developed cancer anyway would either have not reproduced, or developed some mechanism to fight this cancer or live with it quite fine, thereby giving us a mechanism we can leverage today (besides apoptosis). This hypothetical mechanism still requires VLC/INSINH, of course.
On a side note, have you, Richard and Eugene, thought of associating with NuSI, or are already?
I agree that is consistent with the general idea. We are in contact with NuSI and pretty much working in parallel on the same mission and we encourage everybody to join or donate.
Martin, thank you for your comment. And yes, we’re definitely on board with NuSI.
What strikes me is the possibility that the results of the proposed test will be only slightly encouraging, because only part, or one type, of the natural selection pressures are being duplicated.
It seems reasonably clear that part of the genesis of many human cancers is the separation of the cell from (or reduced connections to) its neighbors as a result of less than optimum Vitamin D levels. It has been reasonably proposed that these reduced connections leads to NS pressures on the less connected individual cells. Considering that increased Vitamin D seems to show some effect on slowing or stopping cancer growth, whatever the precise mechanism, probably by enhancing the connections of the cells to surrounding cells, the combination of reduced CHO and increased Vitamin D might be even more effective.
Underlying all of this is of course the idea that cancer is not some evil and demonic modern plague, but rather a predictable result of modern “nutrition” and of modernity itself (not enough skin exposed to sunlight…).
All of these are part of the picture but you have to remember that cancer is a cellular process that applies to all living organisms. Humans are not the only ones who develop cancers.
Dear Richard:
Vitamin D could certainly play a role in cancer growth and inhibition as it is involved both in insulin sensitivity and in immune regulation. There are many avenues to explore.
And what about protein restriction as well ?
Recent papers I have seen involving the benefits of calorie restriction for example are suggesting that it is not the calories but the protein restriction that is making the difference.
eg Luigi Fontana is investigating that short term fasting alone had little effect on IGF-1 , But when protein restriction was introduced IGF-1 levels fell significantly lower.
I agree Vitamin D looks like an important consideration too. The combination could be powerful
I believe Richard is referring to Cedric Garland’s DINOMIT theory.
Where D is the disjunction occurring because of a lack of vitamin D.
A recent paper also highlighted that Vitamin D is directly involved with Autophagy .
Could a Keto diet with lower but still adequate protein be better than a keto diet with slightly more protein intake ?
Fontana recently found protein had to be below 10% of calories , or .8 grams per kilogram of body weight., To get the reductions in IGF-1
One of his papers from 2008
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2673798/
“”These findings demonstrate that, unlike in rodents, long-term severe CR does not reduce serum IGF-1 concentration and IGF-1 : IGFBP-3 ratio in humans. In addition, our data provide evidence that protein intake is a key determinant of circulating IGF-1 levels in humans, and suggest that reduced protein intake may become an important component of anticancer and anti-aging dietary interventions.”
All of these may be part of the picture and are among the things we would like to do in future studies.
I agree that understanding the role of dietary protein is extremely important and reducing the protein content in our ketogenic diet would be fascinating to study. I’m sure you’re aware that the appropriate amount of protein in the diet has a long history of controversy. Even in the Calorie Restriction community recommendations are all over the place (from 20 to 150 grams a day, e.g.). So the study you quote is very interesting.
It’s exceptionally hard to tease out what’s important in living (i.e. very non-linear) systems since the familiar scientific method, which has worked so well for so long, is nontheless accustomed to changing only one variable at at time. The problem becomes even more challenging with ‘protein’, which itself is a remarkably heterogeneous combination of amino acids. I mention this because I was intrigued by an article in Nature 2000: doi:10.1038/nature08619 “Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila” by Grandison et al. Their point was that in Drosophila, methionine restriction alone mimicked the effects of calorie restriction. Of course fruit flies aren’t humans (and humans have considerably heterogeneity among themselves), but the article highlighted (to me, anyway) how little we really know about what the relevant parameters are when we say we should restrict protein.
Hi Dr. Fine and Dr. Feinman,
Thanks for these very illuminating posts about your research. As a 1 year breast cancer survivor, I appreciate them greatly.
I wanted to relate an n=1 experiment in ketogenic diets on hormone positive, aggressive, late stage breast cancer.
I’m unfortunately still in the obese category and have eaten low carb for most of 12 years. When I developed Breast Cancer, my Vit D level was 19 at diagnosis and is still only at 29 despite 5000iu supplementation through therapy.
I started researching like mad and came to the conclusion that low carb was better than high carb for me. I went against all the modern cancer literature that recommended eating a mostly vegetarian diet filled with “healthy whole grains” and a balanced approach to nutrition that includes lots of healthy fruits. It was a high stress and confusing time to decide on a diet for the duration of treatment (short term) and for life (long term).
I knew I may have potentially made the cancer mutate and become stronger through my low carb diet – there is a study out there discussing how cancer cells can become adapted to ketone bodies as fuel and it can potentially make the cancer become more aggressive. You explain it much better in this post!
My oncologist was encouraged and admittedly surprised by the pre-surgery results of neo-adjuvant chemo. He told me that most cancers of my type are “lazy eaters” and don’t typically respond with gusto to chemo. But my cancer had an over 60% reduction in size of the primary tumor (from almost 3cm to less than 1cm at time of surgery).
I’m now in the process of reconstructive surgeries and am taking AI (Aromatase Inhibitor – Femara) anti-hormone therapy for 5-10 years. I don’t have high odds that I will remain rating NED (No Evidence of Disease) but my oncologist tells me there is a fighting shot at longterm remission or living cancer free because of the response we saw with chemo.
Thanks to both of you for your dedication and hard work as healers and teachers!
Dear Susie:
Thank you for your comment and your story. Your experience sounds harrowing, at least for awhile.
I’m so glad that you have had such a good response to the neo-adjuvant therapy in reducing the size of your tumor so well before surgery. As you say, from the point of view of the dietary component you may be only an n=1 anecdotal case, but there’s nothing wrong with that, since you’re the n=1 who’s doing better.
I sincerely hope that you will continue to get better and stay healthy for a long long time.
Other creatures share features of genetics, biology and physiology that show broad similarities with our own. We shouldn’t be too surprised when they suffer conditions of ill-health broadly in keeping with our own.
But Hippocrates gave us a helpful ‘steer’ that perhaps we should look to the circumstance(s) in which ill-health arises. If other creatures show greater incidence of ill-health when subjected to living standards, nutrition, and habitation more closely resembling our own then might there be a valuable clue? Cats and dogs kept as pets show increased incidence of diabetes, say, over their wild counterparts, it is suggested, so might the same be true for cancer?
However I blindly stumbled across the school of holism long before I became aware such a school existed. Where reductionism helps identify specific links (or components of specific links) in the chain of causality, holism helps piece several links together, sometimes to good effect. Holism does not provide certainty, but it can lend additional confidence or insight via associations and synergies.
It is interesting, I think, that Cosmologists and Geologists (macrogeologists) increasingly model their logic and reasoning along ‘Darwinian’ lines. Each study something that represents succession of circumstance, ie. transition from one circumstance to the next, that was acted upon by some selection pressure(s) of some kind or other. The humanities, social science, and to an extent medical science seem slow to catch up. Permit me to explain.
There are two broad swathes to science. The study of things natural .. .. and the study of things arising from the behaviour, beliefs, or constructs of man.
The physical and natural sciences have to presume ‘causality’ is omnipresent founded upon a philosophical standpoint, whereas social science ought to be confident, because the omnipresence of links between cause and outcome is assured by the very dominion studied by the discipline. It is, I find, such a waste that social science cannot reliably pin down the nature of causality where causality is evidently (predominantly) man-made. Medical science has a foot in both camps.
After several thousand hours of reflection I have adopted an integrated evolutionary outlook spanning from ‘big-bang to banking crisis’. In this integrated view any circumstance in any given time represents an advance upon an earlier circumstance. The ‘trajectory’ that describes the change from former circumstance to the next was consistently guided by selection pressures, be they laws of physics, human preference, or human actions driven by authentic or false belief. This, I feel, is no great shakes, but it does refresh the mind of the omnipresent nature of the link between ”cause and effect’, and it points the (open) mind to the real nature of the power of the almighty. Then crucially .. ..
.. .. for incidence of a disease to favour a particular time (modernity) and places (‘the developed west’) or other such ‘dominions’, then rightful regard for the power of the ‘almighty’ directs that the causes must reside in reasonable proximity to the times, places, and dominions in which the outcomes that result are witnessed.
So I wanted to trouble to draw attention to very pertinent sentiments exceedingly well expressed, hence I shall quote them:
I am a layman of limited ability so I crave explanations that sit within the limits of my abilities. ‘My working explanation’ for cancer runs to this:
Cancer in common with many other chronic conditions of ill-health (such as T2DM f.i.) is evidence of a physiology performing under the duress of some stress or other. In keeping with comments I side with the potential value in seeing these physiological ‘stressors’ as being plural by nature and residing somewhere in ‘modernity’. I approach curiosity via several avenues.
* Physiologically speaking high oxidative stress, inflammation, immuno-impediment, and low anti-oxidant status would seem to describe one line linking to stress.
* Evolutionary speaking the big league trends in the evolution of the proto-human and human, and the nature or ‘trajectory’ of co-evolution with the (proto) human diet, points to the highly glycaemic nature of the modern diet – a facet describable by often being low in soluble fibre, low in fat, and trending to being higher in CHO and for those CHOs to be more highly refined or processed. There is also the issue of fat substitution (PUFAs for SaFAs).
*Behaviorally speaking I think the dogmatic adherence to a false medical hypothesis founded upon at least two fatal errors and an outright deception (the lipid hypothesis founded by Dr Keys) that has resulted in more harm than good.
* ‘Finance’. The quirky nature of fiat currency, if actively studied (and it is rare that it ever is) constitutes a selection pressure in its’ own right that compounds some of the issues indicated above. A visit to the money sceptic authors and advocates of alternate or complementary currencies, will be rewarded by a major transition in cognition.
* Modernity. I agree: Isolation from, or diminished exposure to, natural factors like sunlight is an unhelpful compromise in our modern world. Greatly reduced access to the healthful ‘free electrons’ that ‘ground’ provides (isolation) ought not to be overlooked – for the biochemical and bio-electrical nature of life is the very epitome of ‘radical chemistry’ that thrives upon the orchestrated movements of pi-electrons.
* ‘Novelty’. Exposure and bodily assimilation of known carcinogens has to be a factor. Old-fashioned features of modern foods are unlikely to result in a modern plague, but new-fangled molecules and additions without evolutionary basis or justification, like aspartame, hydrogenated fats or esterification of oils, say, might be promotional via absence of suitable former exposure and corresponding adaptation.
A look at the process of money supply, and contemplation of the ‘ecology’ that results, will remove the veil concealing why social science (and to some extent medical science) does such a goddam awful job of arriving at consensus, and/or of reliably explaining causality, when we know, in social science at least, that causality is absolutely implied and must trace, by definition and distinction, to the behaviour, beliefs, or constructs of man himself. Lamentably, social scientists do a credible job of ignoring the ‘ecology’ that arises from one particular ‘medium of exchange’, and although a leap requiring qualification, our health suffers as a result. And science suffers too for, in keeping with observations of E F Schumacher, the ‘science of manipulation’ is rewarded financially for trumping the altruism inherent in the more morally upstanding ‘science of understanding’.
HIPPOCRATES
Hippocrates and Rob Stein of the Washington Post are better equipped than many a contemporary practitioner to direct that modern life is ravaging our immune systems.
Mechanisms are expected to be similar among different organisms but the cell does not know whether or not a stimulus is man-made notwithstanding expert opinion (Hippocrates and Rob Stein ? Ha).
Dear Christopher Palmer:
Civilization indeed has produced problems contributing to diseases, as discussed. However there isn’t a one way street. The incidences of many diseases have been reduced due to sanitation, plumbing, vaccines, hydration, and other public health measures and medical practices. Disease and health are dynamic processes occurring over periods of time and place and depend on, among other things, our powers of reason and discourse (linked to civilization, to Hippocrates and to this conversation) to continue to solve problems. May the dialogue continue.
Dear Dr. Fine:
Under the low-CHO diet, the cancer cells no longer get enough to eat, so they’re not proliferating. Thus, they quit absorbing the labeled glucose and they don’t show black in the PET scan.
If they’re not proliferating, are they dying? Or just quiescent? Many of the anti-tumor therapies don’t target cancer per se, they target rapidly-dividing cells. So would a cancer cell, no longer dividing, be rendered less vulnerable to radiation and chemotherapy because it’s just sitting there biding its time?
Of course, if the cancer cells are dying in the low-insulin environment, this is not a problem. Are they dying? Does low-CHO render them aptotic? Or are they just shriveling away?
I will send this on to Dr. Fine but I think it is not they do not get enough to eat but but that part of what’s available (ketone bodies) is disrupting the metabolism of what they do have.
Dear Adam:
Thank you for your insightful comments. You’re right, but only in part. There are multiple mechanisms of insulin inhibition’s action. Some are consistent with static effects inhibiting proliferation, as you describe, others with lytic (apoptotic-killing) effects. The overall efffect can’t yet be predicted even in priniciple because cell signaling effects a) have not yet been effectively quantitated; b) are wildly non-linear since all the signaling feeds back on itself, and c) will differ from cancer to cancer, even within cancers of the same histology.
Some interesting insight from this paper
http://www.nutritionandmetabolism.com/content/8/1/41
Recently, Guevara-Aguirre et al reported on 99 Ecuadorian individuals with Laron syndrome due to growth hormone receptor (GHR) deficiency and congenital insulin-like growth factor-1 (IGF-1) deficiency who did not develop type 2 diabetes (T2D) and were almost free of cancer, in contrast to their healthy relatives with normal insulin/IGF-1 signaling (IIS) [1].
A recent worldwide survey of Steuerman et al demonstrated that none of 230 individuals with Laron syndrome developed cancer [2]. Laron syndrome is a very informative experiment of nature and uncovers the link between low IIS and the related protection from diseases of civilization in contrast to exaggerated IIS induced by Western diet as shown in Figure 1.
Thanks for this link. Looks interesting. Will check it out.
[…] carb is that? The typical US diet provides 250 to 400 grams of carbohydrates daily. According to Dr. Eugene Fine, who recently did a small human trial on low-carb diets for cancer, you need to eat 50 grams or […]
[…] du vill läsa mer om det så finns lite länkar här, här och här som du kan plöja […]
I’ve been pursuing inquiry into preventive approaches for a particular form of sarcopenia that is due to muscle unloading, for possible ketosis benefit. A long shot due to mTor inhibition in the ketotic state, but considered by analogy with mammalian hibernation that preserves muscle mass after disuse. Anyway, pulling in reading, spotted this re cancer, and the cancer-host system. An in vitro, and in vivo xenograft, tumor study.
1. Cancer Metab. 2014 Sep 1;2:18. doi: 10.1186/2049-3002-2-18. eCollection 2014.
Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer
cachexia.
Shukla SK(1), Gebregiworgis T(2), Purohit V(3), Chaika NV(1), Gunda V(1),
Radhakrishnan P(1), Mehla K(1), Pipinos II(4), Powers R(2), Yu F(5), Singh PK(6).
BACKGROUND: Aberrant energy metabolism is a hallmark of cancer. To fulfill the increased energy requirements, tumor cells secrete cytokines/factors inducing muscle and fat degradation in cancer patients, a condition known as cancer cachexia. It accounts for nearly 20% of all cancer-related deaths. However, the mechanistic basis of cancer cachexia and therapies targeting cancer cachexia thus far remain elusive. …Keeping in view the significant role of metabolic alterations in cancer, we
hypothesized that a ketogenic diet may diminish glycolytic flux in tumor cells to alleviate cachexia syndrome and, hence, may provide an efficient therapeutic strategy.
RESULTS: We observed reduced glycolytic flux in tumor cells upon treatment with ketone bodies. Ketone bodies also diminished glutamine uptake, overall ATP content, and survival in multiple pancreatic cancer cell lines, while inducing apoptosis. A decrease in levels of c-Myc, a metabolic master regulator, and its recruitment on glycolytic gene promoters, was in part responsible for the
metabolic phenotype in tumor cells. Ketone body-induced intracellular metabolomic reprogramming in pancreatic cancer cells also leads to a significantly diminished cachexia in cell line models. Our mouse orthotopic xenograft models further confirmed the effect of a ketogenic diet in diminishing tumor growth and cachexia.
CONCLUSIONS: Thus, our studies demonstrate that the cachectic phenotype is in part due to metabolic alterations in tumor cells, which can be reverted by a ketogenic diet, causing reduced tumor growth and inhibition of muscle and body weight loss. PMCID: PMC4165433
PMID: 25228990 [PubMed]
Dear Rob,
Altered cancer cell metabolism after supplemental ketone bodies may be the reason for reduced cachexia as the authors have elegantly demonstrated in their cell culture study. They follow a durable tradition along these lines dating to the 1980’s. Tisdale et al (1) have a couple of citations among the authors’ references, but their work on this dates back to the mid-1987 when they tested whether ketosis might improve cachexia and found that it did but, incidentally, also reduced tumor size. This is a valuable contribution to the literature. It could also lead, perhaps, to improved identification of more conveniently measured specific cytokines responsible for cachexia.
I don’t know the answer to your more general question whether ketosis would inhibit sarcopenia in other situations. The upstream cause of ketosis, i.e. the inhibition of insulin by carb restriction (and consequent downstream inhibitory effects on mTOR, etc.) could be a problem. But Veech (2) describes how efficient ketone bodies are as fuels. And Volek and Phinney (3) point out how the highest levels of athletic performance are enhanced by ketosis, from activities as diverse as endurance running to power lifting. So you may be on to something.
1. Tisdale MJ, Brennan RA, Fearon KC: Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer 1987, 56(1):39-43.
2. Veech RL: The therapeutic implications of ketone bodies… Prostglanding, Leukotrienes and Essential Fatty Acids, 2004: 70:309-319
3. The art and science of low carbohydrate performance: Jeffrey S. Volek and Stephen D. Phinney, Beyond Obesity (publishers); 2012
Eugene,
I appreciate your kind reply, and the information is useful. Thank you. I had not known about Tisdale’s work, and will hunt that down now. The bibliography concerning promising clinical applications of the KD and/or ketone precursor administration is growing quite large. Makes sense, I guess, when an intervention can lead to fundamental alterations in cell energy metabolism as well as other regulatory and signaling functions.
That was all I was planning to say, then this paper below turned up. It seems to belong in this thread of discussion, so I’ll post it here. Cell cultures, in vivo xenografts, metformin, and KD.
PLoS One. 2014 Sep 25;9(9):e108444. doi: 10.1371/journal.pone.0108444. eCollection 2014.
Mechanisms by Which Low Glucose Enhances the Cytotoxicity of Metformin to Cancer
Cells Both In Vitro and In Vivo.
Zhuang Y, Chan DK, Haugrud AB, Miskimins WK.
Different cancer cells exhibit altered sensitivity to metformin treatment. Recent
studies suggest these findings may be due in part to the common cell culture
practice of utilizing high glucose, and when glucose is lowered, metformin
becomes increasingly cytotoxic to cancer cells. In low glucose conditions ranging
from 0 to 5 mM, metformin was cytotoxic to breast cancer cell lines MCF7,
MDAMB231 and SKBR3, and ovarian cancer cell lines OVCAR3, and PA-1. MDAMB231 and
SKBR3 were previously shown to be resistant to metformin in normal high glucose
medium. When glucose was increased to 10 mM or above, all of these cell lines
become less responsive to metformin treatment. Metformin treatment significantly
reduced ATP levels in cells incubated in media with low glucose (2.5 mM), high
fructose (25 mM) or galactose (25 mM). Reductions in ATP levels were not observed
with high glucose (25 mM). This was compensated by enhanced glycolysis through
activation of AMPK when oxidative phosphorylation was inhibited by metformin.
However, enhanced glycolysis was either diminished or abolished by replacing 25
mM glucose with 2.5 mM glucose, 25 mM fructose or 25 mM galactose. These findings
suggest that lowering glucose potentiates metformin induced cell death by
reducing metformin stimulated glycolysis. Additionally, under low glucose
conditions metformin significantly decreased phosphorylation of AKT and various
targets of mTOR, while phospho-AMPK was not significantly altered. Thus
inhibition of mTOR signaling appears to be independent of AMPK activation.
Further in vivo studies using the 4T1 breast cancer mouse model confirmed that
metformin inhibition of tumor growth was enhanced when serum glucose levels were
reduced via low carbohydrate ketogenic diets. The data support a model in which
metformin treatment of cancer cells in low glucose medium leads to cell death by
decreasing ATP production and inhibition of survival signaling pathways. The
enhanced cytotoxicity of metformin against cancer cells was observed both in
vitro and in vivo.
PMID: 25254953
[…] Richard Feinman, PhD: Dr. Feinman is a professor at the State University of New York. He has collaborated multiple times with Dr. Fine. Dr. Fine wrote two blog posts on Dr. Feinman’s site: Part 1 and Part 2. […]
[…] Since Warburg’s work, interrupted by a world at war, many studies of human populations have found that high levels of insulin are associated with increased risk of many cancers (but not all cancer. Slow-growing prostate cancer, for example, is apparently not driven by glucose.) […]